Levine Cancer Institute Department of Biostatistics and Data Sciences
The Atrium Health Levine Cancer Institute (LCI) Department of Biostatistics and Data Sciences provides design, rigor and support for research projects whose goal is to identify and advance cancer therapeutics.
At its core, our work is about providing researchers with the results they need to better inform whether cancer treatments are working and should be continued, are not working and should be discontinued, or if a new form of treatment should be pursued – all with the goal of advancing outcomes so our patients can live their best lives.
Our team collaborates on cancer research in 4 ways:
- Biostatistics: We provide study design, sample size estimation, protocol development, statistical analysis, regulatory and compliance reporting, and manuscript and grant writing support to LCI investigators.
- Bioinformatics: We manage, process and analyze genomics big data to identify biomarkers for cancer diagnostics, therapeutics and prognosis.
- Quality Assurance Monitoring: We ensure protection of the rights, safety and well-being of clinical study participants and that study data are accurate, complete and reproducible per the study protocol, federal regulations, good clinical practices and local standard operating procedures.
- Observational Research Data Core: We collect real-world clinical data and patient-related outcomes from electronic health records, cancer registries and other data sources for observational studies in cancer research.
For LCI partners interested in collaborating with our team, please submit your research concept to the Investigator-Sponsored Research Portal.
LCI Biostatistics
The LCI Biostatistics team collaborates with LCI researchers on investigator-initiated research (IIR), which includes clinical trials, observational studies, translational research, omics studies and health economics studies.
The Biostatistics team supports all phases of research, including the initial concept phase, development process, oversight phase, final analyses and ultimately the publication of results. Our biostatisticians:
- Provide study design, sample size estimation, protocol development and statistical analysis
- Collaborate with investigators on reporting for regulatory and funding entities, as well as manuscript and grant writing
- Serve as co-investigators on research grants
- Serve on the LCI Data and Safety Monitoring Committee and the LCI Protocol Review and Monitoring Committee
- Provide formal biostatistics education at LCI and mentor junior investigators
Meet the Team
James Symanowski
- Ferdous Ahmed, PhD – Senior Biostatistician
- Danielle Boselli, MS – Senior Biostatistician
- Erin Donahue, PhD – Senior Biostatistician
- Sachin Mhatre, PhD – Postdoctoral Research Associate
- Myra Robinson, MS – Senior Biostatistician
- Allison Verbyla, MS – Intermediate Health Services Researcher
- Hang (Laurel) Zeng, MS – Intermediate Biostatistician
- Karl Dykema, BA – Senior Biostatistician
Administrative Support
- Katrina Scott, AAB – Senior Staff Assistant and LCI Data Safety Monitoring Committee Coordinator
LCI Bioinformatics
The LCI Bioinformatics team collaborates with physicians, laboratory scientists and biostatisticians to provide expertise in genetics, genomics, bioinformatics and high-dimensional computations – all with the goal of identifying new biomarkers that can enhance therapeutics and precision oncology care at LCI.
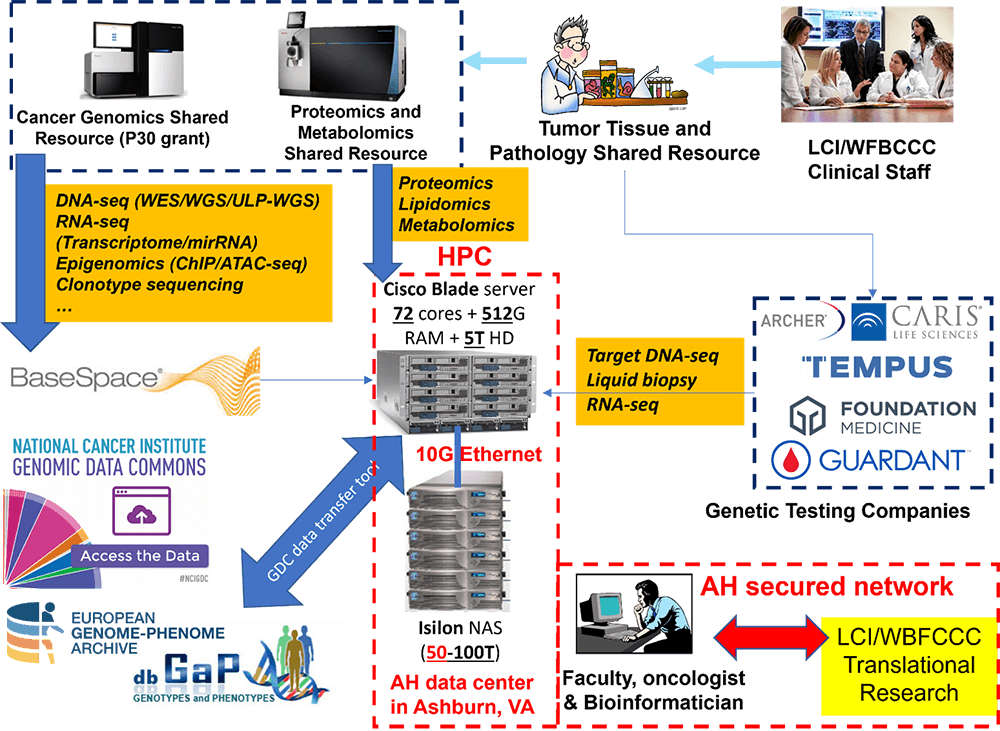
The primary function of this team is to design and analyze genomics big data, including next-generation sequencing and epigenetics, to provide interpretable results and graphical visualizations for use in grant applications and research articles.
In addition, the Bioinformatics team works with Information and Analytics Services to develop and maintain a high-performance computing framework. This cutting-edge framework not only streamlines bioinformatics analysis, but also secures genomics big data, enhancing the reliability, capability and capacity of bioinformatics services and genomics data management for researchers across LCI.
Meet the Team
- Jenny Chen, MS – Senior Biostatistician
- Sharvil Desai, PhD – Senior Biostatistical Analyst
LCI Quality Assurance Monitoring
The LCI Quality Assurance (QA) team is responsible for ensuring patient safety and that the rights and well-being of all human subjects involved in research at LCI are protected.
The QA team ensures that reported trial data are accurate, complete and verifiable from source documents. The team also works with principal investigators, sponsor investigators and study teams to make sure they are adhering to all protocols, federal regulations, good clinical practices and local standards of practice.
The QA team conducts sponsor-required monitoring for all relevant LCI investigator-initiated studies, works with stakeholders to review protocols in the development of new studies and reviews data for Food and Drug Administration inspections or co-op audits.
Meet the Team
- Michelle Anderson, BA – Senior Clinical Site Monitor
- Andee Fox, MPH, CCRP – Clinical Site Monitor, Level II
- Eryn McCraw, BS, CCRP – Senior Clinical Site Monitor
- Sandra Sylver, BS, CCRA – Senior Clinical Site Monitor
- Caleb Suddreth, BS, CCRC – Clinical Site Monitor, Level II
- Sarah Teague, BS, CCRP, CCRA – Clinical Site Monitor, Level II
LCI Observational Research Data Core
The LCI Observational Research Data Core (LORDC) team collects real-world clinical treatment and outcomes data from LCI patients. This data provides a resource for sponsored investigators and biostatisticians to conduct observational research. The LORDC team includes systems engineers and data coordinators who extract, transfer, process, load and audit patients’ electronic health records and outcomes data from various sources into the LORDC database, a state-of-the-art web-based application that securely stores patients’ real-world clinical data and outcomes to be used for observational cancer research. The LORDC team also collaborates on protocol development, abstract preparation and manuscript writing.
Meet the Team
- Stephanie Begley, MS – Oncology Research Specialist
- Deanna Johnson, BS – Oncology Research Specialist
- Shanice Borden, MS – Oncology Research Coordinator
- Rachel Russek, MS – Oncology Research Specialist
- Markeeta Garner, BS – Oncology Research Specialist
- Deepthi Krishnan, BS – Intermediate Application Specialist
- Nicole Serapin, BS – Oncology Research Coordinator
- Erica Wade, BS – Oncology Research Coordinator
- Zoe Mulligan, BS – Oncology Research Coordinator
