Valve and Structural Heart Disease
Sanger Heart & Vascular Institute is pioneering innovative approaches and devices to redefine structural heart and valve care.
Recent Highlights
Sanger became the first center in the Carolinas to use the new transcatheter Medtronic Evolut™ FX TAVR system to treat patients with aortic stenosis.
Multidisciplinary Valve Care
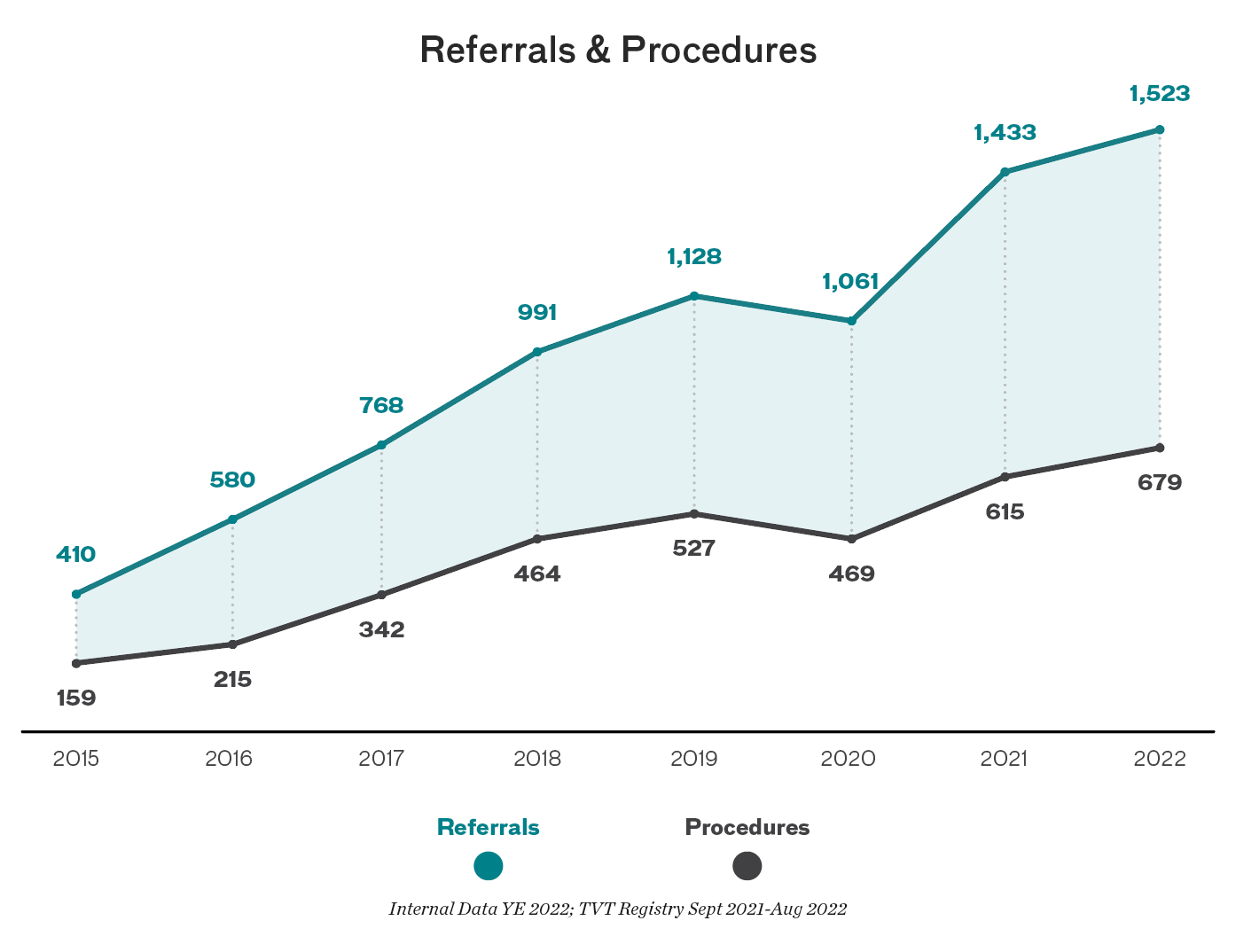
For our new Valve Center, we created an innovative, multidisciplinary model that streamlines care and ensures timely access to key tests and images. This shortened the time it takes from patient referral to workup completion by more than three weeks, to an average of 45 days.
Clinical Trials and Innovative Technologies
- TRILUMINATE: Sanger was 12th in the world to enroll a patient in this trial of the TriClip™ transcatheter valve repair system in symptomatic patients with severe tricuspid regurgitation.
- AltaValve/4C: We performed the 13th case in the U.S. using this transcatheter mitral valve replacement technology.
- NeoChord: Sanger successfully performed the first-in-human transcatheter mitral valve repair via a trans-septally delivered NeoChord device.
- 4D Intracardiac Echocardiography: Sanger was one of the first 20 U.S. sites to utilize this new technology for MitraClip™, TriClip and left atrial appendage cases.
- Evolut FX: We were the Carolinas’ first center to use this delivery system for TAVR.
- Cephea: We were the second U.S. center to use this transcatheter mitral valve replacement technology.
- EVOQUE: We are the only center in the Carolinas performing transcatheter tricuspid valve replacement with EVOQUE.
- ACURATE neo2™: A clinical trial utilizing an innovative valve that may be a good option for certain patients, including those with high pacemaker risk.
Awards and Recognition
-

-

STS/TVT 3-Star Rating
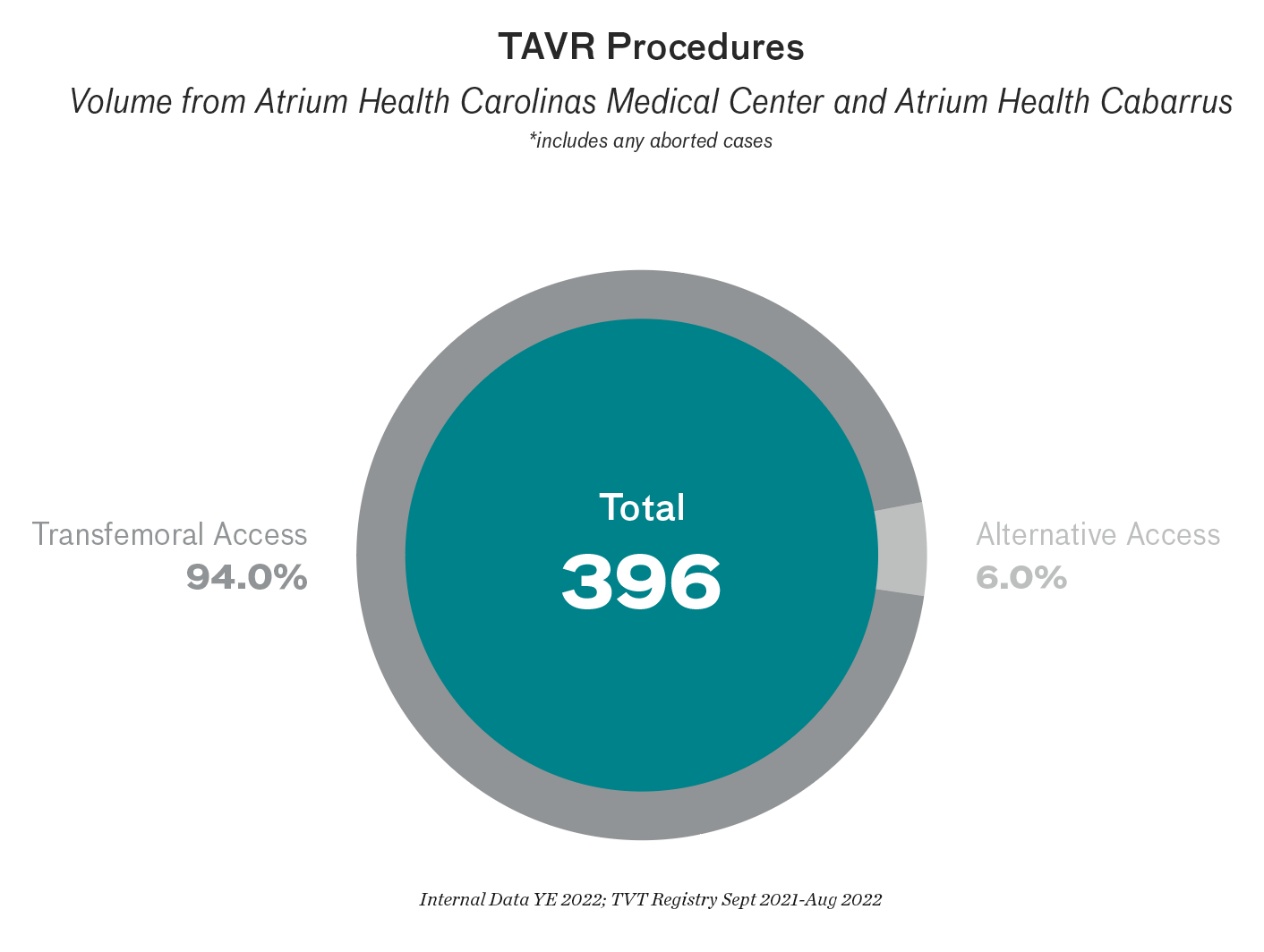
TAVR
Mitral Valve
-
59
Mitral and Tricuspid Valve Procedures
Internal Data, YTD September 2022 Annualized -
>90.0%
Mitral Valve Acute Procedural Success - MR grade ≤ 2+
TVT Registry, September 2021 - August 2022 -
0.0%
30-Day Observed Mortality Rate Unadjusted
Benchmarks:
50th Percentile: 0.0%
90th Percentile: 0.0%
TVT Registry, September 2021 - August 2022 -
0.0%
Mitral Valve In-hospital Mortality Rate Unadjusted
Benchmarks:
50th Percentile: 0.0%
90th Percentile: 0.0%
TVT Registry, September 2021 - August 2022 -
0.0%
Mitral Valve Stroke Rate
Unadjusted
Benchmarks:
50th Percentile: 0.0%
90th Percentile: 0.0%
TVT Registry, NCDR
Structural Heart and Valve
Left Atrial Appendage Occlusion
-
>90.0%
Patients Free of Anticoagulation At 6 Months
LAAO Registry, January - June 2022
Publications and Presentations
Characterization of cerebral embolic capture using the SENTINEL device during transcatheter aortic valve implantation in low to intermediate-risk patients: the SENTINEL-LIR study. Kawakami R, Gada H, Rinaldi MJ, et al. Circ Cardiovasc Interv. 2022;15:e011358.
Dual Antiplatelet Therapy Discontinuation, Platelet Reactivity, and Adverse Outcomes After Successful Percutaneous Coronary Intervention. Redfors B, Kirtane AJ, Liu M, Musikantow DR, Witzenbichler B, Rinaldi MJ, D. Metzger C, Weisz G, Stuckey TD, Brodie BR, Ben-Yehuda O, Mehran R, and Stone GW. J Am Coll Cardiol Intv. 2022 Apr 15 (8) 797–806.
IAGS 2022 Summary Document. Abbott JD, Adams G, Alaswad K, Anderson HV, Arain S, Aronow H, Bersin R, Brown C, Collins T, Cowley M, Fischell T, Garratt K, Greenbaum A, Grines C, Helmy T, Henry T, Lerman A, Lucas V, Magd A, Marshall J, Meduri C, Mustapha J, Nikol S, Ohman E, O’Neill W, Rinaldi MJ, Sathananthan J, Smalling R, Szerlip M, Vetrovec G, Zidar J. J Invasive Cardiology. E1-50. Vol. 34 Epub 2022 June 24.
Cardiac Computed Tomography and Magnetic Resonance Imaging of the Tricuspid Valve: Preprocedural Planning and Postprocedural Follow-up. Lopes B, Hashimoto G, Bapat V, Sorajja P, Scherer M, Cavalcante J. Intervent Cardiol Clin 11 (2022) 27-40.
Contemporary, Real-World Outcomes Of 5,000 Patients with Secondary Mitral Regurgitation Treated with MitraClip: Results from the COAPT Post Approval Study. Makkar R, Naik H, Atmakuri S, Mahoney P, Morse A, Yadav P, Batchelor W, Rogers J, Whisenant B, Rinaldi MJ, Hermiller J, Lindenfeld J, Lindman B, Barker B. European Society of Cardiology 2022.
Role of Cardiac CTA in the Evaluation of Transcatheter Edge-to-Edge Tricuspid Repair - the TRILUMINATE Pivotal Imaging CT Sub Study. Cavalcante JL, Lesser J, Khalique O, Lerakis S, Shah D, Adams D, Trusty P, Sorajja P, Scherer M. Abstract-Poster at SCCT 2022 Annual Session.
Adjudicated Echo and Safety Outcomes with the NTW/XTW from the MitraClip G4 System: Results from the Global EXPAND G4 Study. Rinaldi MJ, Rottbauer W, Rollefson WA, Chehab B, Loureiro RE, Grasso C, Kar S, Asch FM, Rodriguez E, Bardeleben S. London Valves 2022.
3D Vena Contracta Area to Measure Peri-Device Leak Following WATCHMAN Device Deployment. Tannenbaum JER, Kott A, Bhave P, Richardson K. Poster Presentation at ASE Scientific Sessions, June 2022.